Describe the Polar Nature of Water
Universidad de Barcelona Summary. The polar nature of water gives it four characteristics that make it a useful medium for living cells.

The Structure And Properties Of Water Introduction To Chemistry
Review the POLAR nature of water.

. Speculate on some of the consequences if ice were always denser than liquid water. Physical properties of water. Due to the polar nature of the water molecule itself other polar molecules are generally able to dissolve in water.
Describe how a charge is distributed on a polar molecule like water. Polarity makes water a good solvent gives it the ability to stick to itself cohesion stick to other substances adhesion and have surface tension due to hydrogen bonding. The opposite is mineral turpentine which is.
O-side negative attracted to H-side positive. The polarity of water molecules means that molecules of water will stick to each other. O is very electronegative hogs electrons is negative.
22U2 Hydrogen bonding and dipolarity explain the cohesive adhesive thermal and solvent properties of water. Explain the relationship between the polar nature of water and its ability to form hydrogen bonds. Because each individual water molecule has both a negative portion and a positive portion each side is attracted to molecules of the opposite charge.
Polar one side positive one negative. Water is distributed unevenly on the earths surface. During which phase of water are all hydrogen bonds broken.
This makes water an extremely potent solvent. Describe the cause and effect of the polar nature of water. Science Biology QA Library Explain why the polar nature of water enables water molecules to be cohesive.
The Polarity of Water. The dipoles do not cancel out resulting in a net dipole. For instance If water was not polar it would be a gas at room temperature and have a tremendously low freezing point making life not possible.
The net effect is a partial dipole where the hydrogens have a partial positive charge and the oxygen atom has a partial negative charge. At the air-water interface the polar nature of water permits it to form a skin over the water exterior sturdy enough to hold up small objects. The water molecule forms an angle with an oxygen atom at the vertex and hydrogen atoms at the tips.
The polar nature of water is a particularly important feature that contributes to the uniqueness of this substance. If the Δχ is between 05 and 16 the bond is considered polar covalent. All the properties of water are related to one another by the polar nature that is caused by the partial electronegativity difference of the hydrogen and oxygen molecules of water molecules.
It can form hydrogen bonds with other elements. How does the polar nature of the water molecule result in the formation of hydrogen bonds between neighboring water molecules. An excellent example of the high polarity of water would be the fact that salt dissolves in water.
Researchers describe the role of water in protein folding Date. Polar substances are covalently bonded substances that contain partially positive and negative charges. A new study describes the.
Water is very polar. Describe where and how water is able to form hydrogen bonds. If the Δχ is between 17 and 20 and if.
Water is a polar molecule which means it can attract other polar molecules. It forms a major solvent and dissolves almost every polar solute. Water is a polar substance which means that any other polar substances will dissolve in it.
So let us have a look at its properties and understand the reason for its significance. As a polar molecule water interacts best with other polar molecules such as itself. The level of polarity in water is extremely high uniquely so.
This fact is known as surface tension. Water is polar because it has a bent geometry that places the positively-charged hydrogen atoms on one side of the molecule and the negatively-charged oxygen atom on the other side of the molecule. Interpret the polar nature of water molecules based on the distribution of shared electrons between oxygen and hydrogen atoms.
September 21 2015 Source. Apr 17 2015. Every water molecule is capable of forming four hydrogen bonds with nearby water molecules 2.
Water has a partial negative charge near the oxygen atom due the unshared pairs of electrons and partial positive charges near the hydrogen atoms. Also why is this characteristic of water so important for plants. Water is made of 3 atoms- one oxygen and 2 hydrogen.
Water is a colourless and tasteless liquid. Water is a polar molecule meaning that there is an uneven distribution of electron density. Water H2O is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other.
H side of the molecule is positive. Explain why the polar nature of water enables water molecules to be cohesive. This is because of the phenomenon wherein opposite charges attract one another.
Hydrogen bonding between water molecules affects the density of water depending on whether it occurs as ice or a liquid. Hydrogens are positive oxygen is negative. This is called hydrogen bonding.
What is polar nature of water. Water is essential for the survival of life on earth. Thehydrogen have an opposite charge to oxygen resulting in a polarcharge.
If the electronegativity difference usually called Δχ is less than 05 then the bond is nonpolar covalent. A water molecule has uneven distribution of charges between different atoms of the different molecules.

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules

Hydrogen Bonding Between Water Molecules H Bonding Is Responsible For Many Of The Important Properties Of Water Such A Hydrogen Bond Water Molecule Molecules
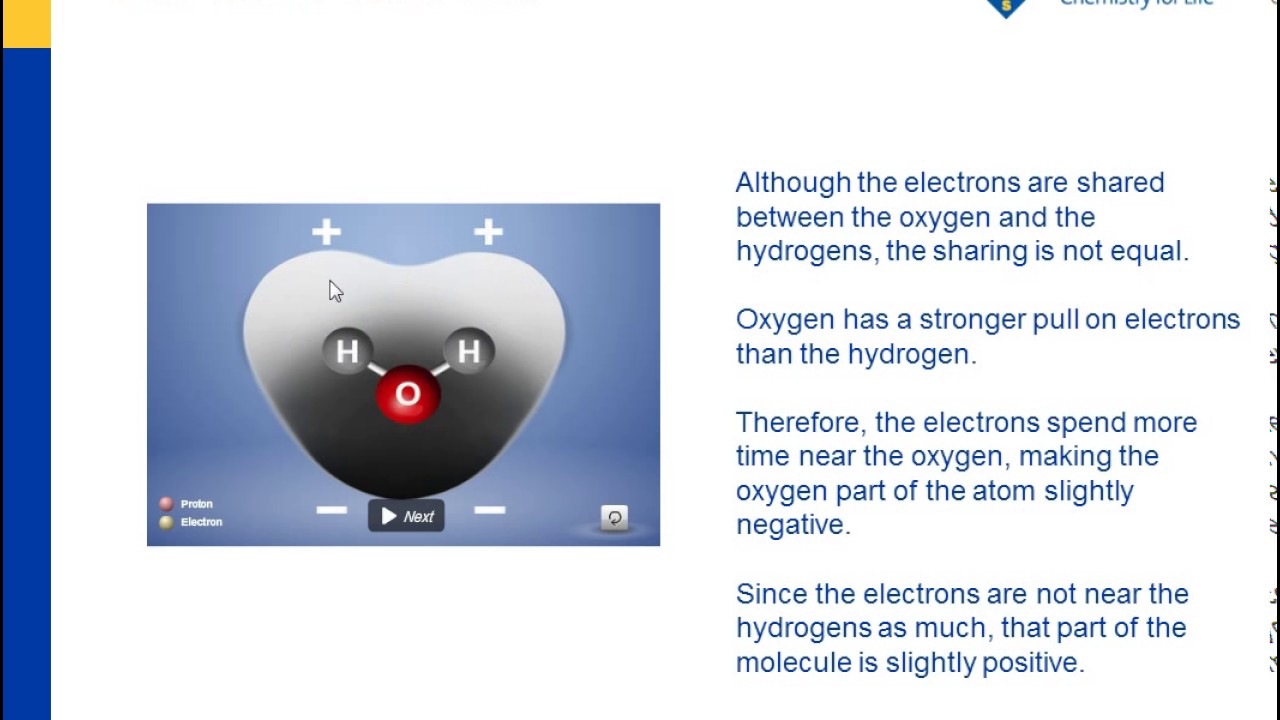
Water Is A Polar Molecule Chapter 5 The Water Molecule And Dissolving Middle School Chemistry
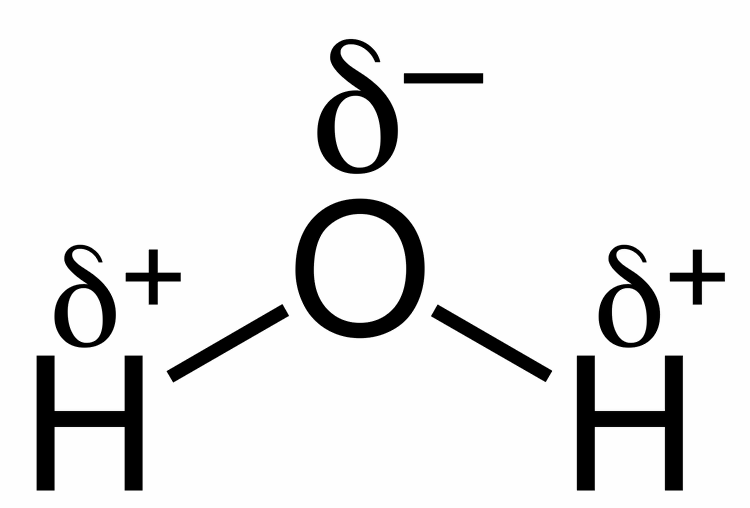
Dipoles Of Water Molecules A Level Biology Revision Notes

2 10 Water Exhibits Numerous Properties And One Of Those Is Cohesion Water Molecules Are Drawn To Each Other Because Of The Polarization Property Quimica

Water Molecules Poster Zazzle Com Water Molecule Education Poster Classroom Posters

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey
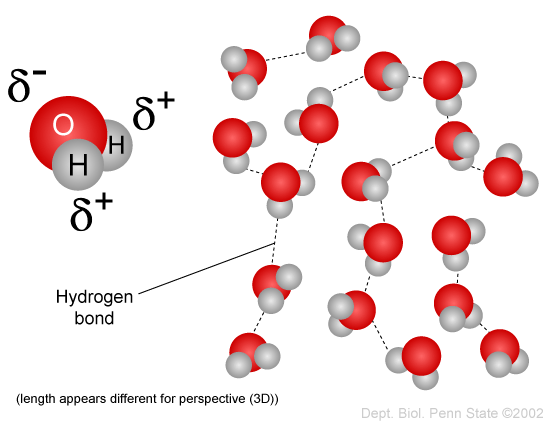
0 Response to "Describe the Polar Nature of Water"
Post a Comment